“This proof-of-concept work represents a new paradigm for electrochemical energy storage,” reads a paper published this week in Nature Materials and led by scientists at Massachusetts Institute of Technology (MIT).
The paper’s authors developed a new class of liquid electrolyte which they say could greatly improve the performance of lithium-ion batteries and supercapacitors – used in some cases to improve performance and extend the lifetime of batteries.
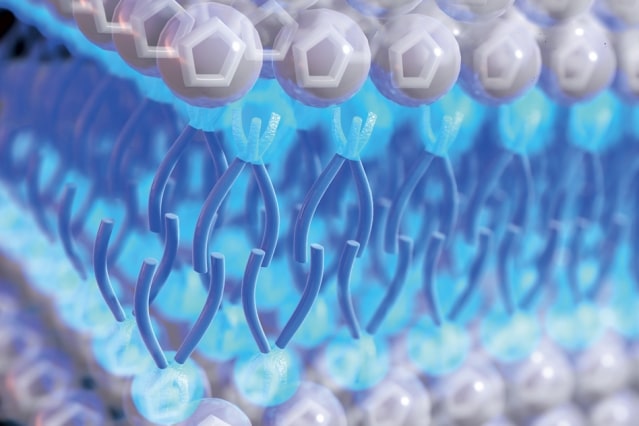
The electrolyte is based on a class of materials known as ionic liquids, which MIT described as “essentially, liquid salts”. The scientists added a compound they said was similar to a surfactant that would be used to disperse an oil spill to the liquid, and found it brought about “new and strange properties” in the liquid which could have several applications for energy storage and other industries.
Enhanced performance
The researchers found the material’s energy density exceeded that of many other electrolytes and it remained highly viscous even at high temperatures, contributing to better safety and stability. T. Alan Hatton, professor of chemical engineering at MIT, explained that was thanks to the way the molecules assembled themselves in a highly ordered structure as they came into contact with another material, such as an electrode.
The ordered structure, according to MIT, helped prevent an issue known as overscreening, where a more scattered distribution of ions at the electrode surface, or a thicker ion multilayer, negatively affects energy storage efficiency.
Likely applications for the technology include high-temperature energy storage, with the researchers pointing out their electrolyte performed even better at high temperatures and was safer and less flammable than others used in lithium batteries as well as in supercapacitors. The researchers speculated their electrolyte could increase energy density four or five times, potentially even allowing it to replace batteries in electric vehicles, stationary storage and consumer electronics.
More to come
The team will now work on other compounds that fit into the new class of materials, which they are calling surface active ionic liquids – SAILs.
“The possibilities are almost unlimited,” said MIT postdoc Xianwen Mao, the paper’s lead author. “It might take a few months or years,” he said, “but working on a new class of materials is very exciting to do. There are many possibilities for further optimization.”
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.








By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.