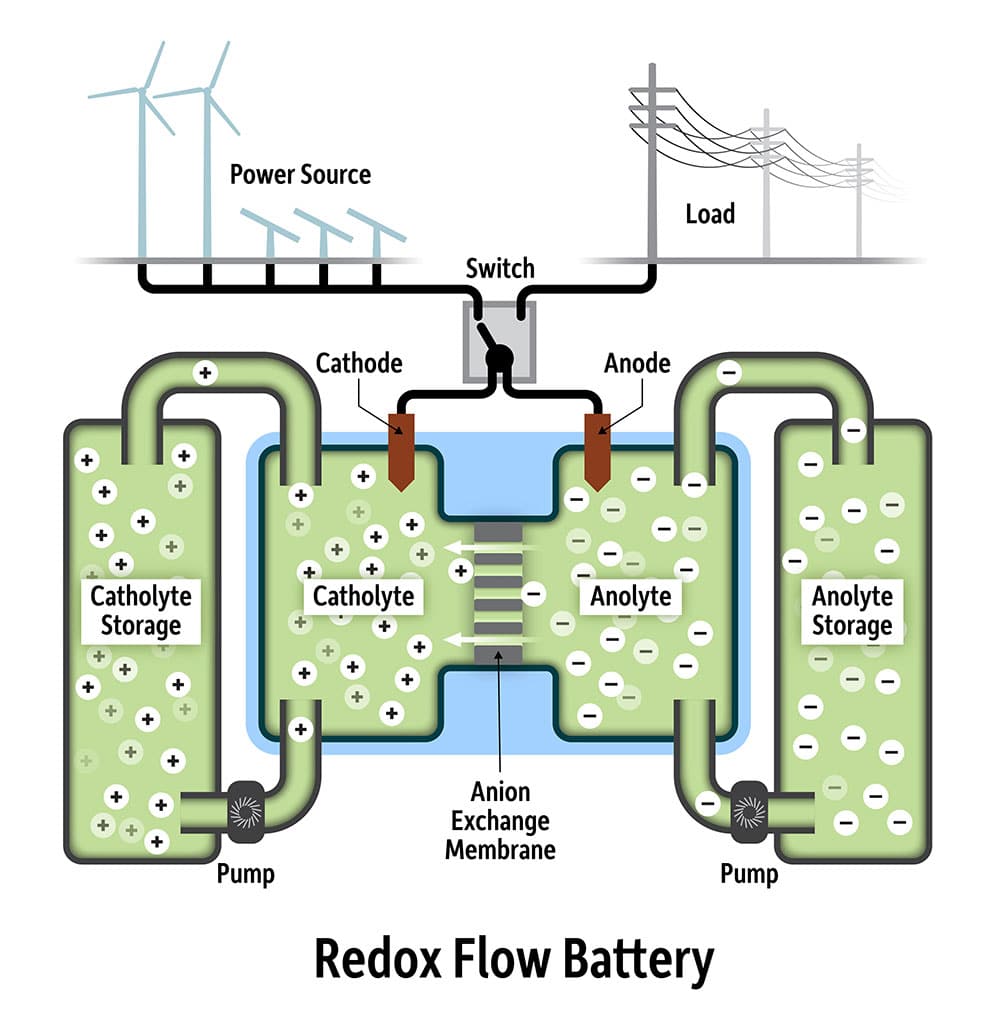
As storage grows into an ever more important part of the energy system, redox flow batteries are often touted as a technology that could rival lithium-ion applications in the large-scale sector.
Finding affordable chemicals that can carry sufficient charge for long periods without degrading, however, remains a barrier to the technology’s widespread adoption – although some commercial projects utilizing redox flow batteries are already underway.
Scientists from the University of Rochester, working alongside colleagues from the University of Buffalo, have discovering a promising compound for use in redox flow batteries, which they say could transform the energy storage landscape.
In research published in the journal Chemical Science, the researchers describe working with a metal-oxide cluster originally discovered by German chemist Johann Spandl, finding that it could be store charge in a redox flow battery, but lacked stability.
By making what is described as a ‘simple molecular modification’, however, researchers were able to increase the stability of the material and double the amount of energy that could be stored in the battery.
“Energy storage applications with polyoxometalates are pretty rare in the literature,” says lead author Lauren VanGelder. “There are maybe one or two examples prior to ours, and they didn’t really maximize the potential of these systems.”
Key to the compound’s potential is the use of readily available materials: “What’s really cool about this work is the way we can generate the ethoxide and methoxide clusters by using methanol and ethanol. Both of these reagents are inexpensive, readily available and safe to use. The metal and oxygen atoms that compose the remainder of the cluster are earth-abundant elements. The straightforward, efficient synthesis of this system is a totally new direction in charge-carrier development that, we believe, will set a new standard in the field.”
The two universities have now applied for a grant from the National Science foundation in order to work on further refining the materials for use in commercial redox flow batteries.
This content is protected by copyright and may not be reused. If you want to cooperate with us and would like to reuse some of our content, please contact: editors@pv-magazine.com.








By submitting this form you agree to pv magazine using your data for the purposes of publishing your comment.
Your personal data will only be disclosed or otherwise transmitted to third parties for the purposes of spam filtering or if this is necessary for technical maintenance of the website. Any other transfer to third parties will not take place unless this is justified on the basis of applicable data protection regulations or if pv magazine is legally obliged to do so.
You may revoke this consent at any time with effect for the future, in which case your personal data will be deleted immediately. Otherwise, your data will be deleted if pv magazine has processed your request or the purpose of data storage is fulfilled.
Further information on data privacy can be found in our Data Protection Policy.